Search productSearch blog
Canine influenza virus (CIV) belongs to the family Orthomyxoviridae and the genus Influenza A virus, which mainly includes two subtypes. The virus has the characteristics of rapid transmission, wide transmission route, high infectiousness, and obvious symptoms. The target of the disease is mainly the epithelial cells of the upper respiratory tract mucosa, with a small proportion appearing in the stomach and intestines. The main manifestations are fever, cough, runny nose, and sometimes high fever with symptoms such as rhinitis, bronchitis, respiratory bleeding, etc. Interstitial pneumonia may also occur in severely affected dogs. The Canine Influenza Virus Antigen Test Kit is used to detect canine influenza virus antigens in dogs’ eye secretions, nasal fluids, and saliva.
This Canine Influenza Virus Antigen Test Kit detects canine influenza virus antigen using a rapid immunochromatographic technique. After samples obtained from eye secretions, nasal fluids, and saliva are added to the test tape wells, the samples are moved along the chromatographic membrane together with a colloidal gold-labeled anti-CIV monoclonal antibody. The T detection line shows red if the CIV antigen is present in the sample. If the CIV antigen is not present in the sample, the T detection line does not appear in color.
The Canine Influenza Virus Antigen Test Kit is able to detect Canine Influenza Virus antigen concentration, demonstrating quantitative results. The test serves only as an aid to the diagnosis, for professional use only.
For rapid screening of Canine Influenza Virus in canine eye and conjunctival secretions, nasal fluid or saliva (CIV).
Note: The above interpretation time is conducted at room temperature 15-30℃. If the temperature is lower than 15℃, the interpretation time should be delayed appropriately. If the temperature exceeds 30 ℃, the results should be read in advance.
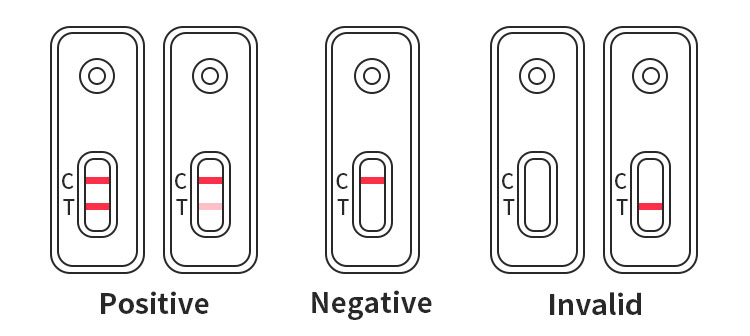
Negative: Only the C line appears as a red color.
Positive: Both T and C lines appear purplish red.
Invalid: No color appears in the C line.
NOTE: Invalid test results can be caused by a variety of reasons and should be re-tested.
Sensitivity ≥ 95%
Specificity ≥ 97.5%
Stability: 2-30 ℃ unopened validity period of 2 years
| Item NO. | Test Cassette | Diluent | Quantitative Pipette | Disposable Swab | Instruction |
| CY-G0012-1 (1 test) | 1 pc | 1 pc | 1 pc | 1 pc | 1 pc |
| CY-G0012-10 (10 tests) | 10 pcs | 10 pcs | 10 pcs | 10 pcs | 1 pc |
| CY-G0012-25 (25 tests) | 25 pcs | 25 pcs | 25 pcs | 25 pcs | 1 pc |
Useful Reviews
This website uses cookies to improve your browsing experience. By continuing to use this site, you accept the use of our cookies. Data collected from this website is processed and stored in the United States.
Submit for consultation
We will reply to you within 24 hours